Australia
Accumulation of minerals including Calcium Bicarbonate (CaCO3) and Chloride (CI)
211 views
Calcium carbonate behaves opposite to household sugar. In other words, it precipitates out of solution and solidifies when in contact with hot surfaces, just like your kettle at home.
As a rule of thumb, the hotter the surface, the more the deposit and in the context of the cooling tower, this is mostly affecting the water-cooled condenser tubes.
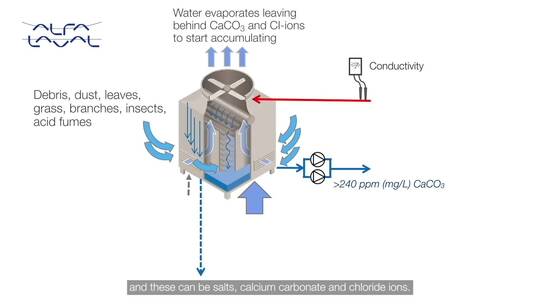
Let’s look into this unwanted mineral calcium carbonate:
If the cooling loop water contains 100 milligrams per litre of calcium carbonate at the start, this concentration is equal to 100 ppm. When half the water in the cooling loop evaporates and is replenished the concentration increases to 150 ppm and this can double or triple over time. Without flushing it can reach unacceptable levels and start causing serious problems.
Similar accumulation happens with chloride ions - they reach excessive levels and cause corrosion to downstream cooling equipment.